Background: Dex is a key component of induction regimens for NDMM despite common toxicities including insomnia and anxiety. The E4A03 randomized trial (Rajkumar, Lancet Oncol 2010) demonstrated that dex dosed at 40 mg weekly outperformed higher-intensity dex and established this regimen as the standard of care for NDMM. In standard practice as well as clinical trials, however, dex doses are often reduced to 20 mg weekly or lower (if not stopped entirely) among older patients or among patients experiencing toxicities. As a first step toward establishing the equipoise of planned dex dose reductions in NDMM, we investigated outcomes with patients from two previous SWOG trials to understand the impact of dex dose reductions on post-induction outcomes.
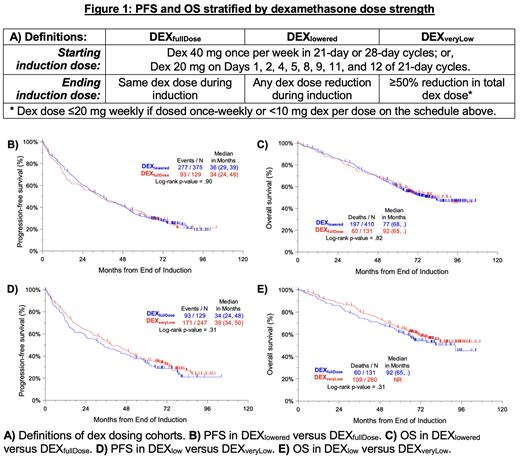
Methods: This was a secondary analysis of data from two completed NDMM studies, S0777 (comparing VRd vs Rd) and S1211 (comparing Elo-VRd vs VRd). For this secondary analysis, we analyzed the subset of patients who completed all eight 3-week cycles of induction (or six 4-week cycles in the S0777 Rd arm). As shown in Figure 1A, we defined DEX fullDose as patients for whom the starting dex dose (either 40 mg once per week or 20 mg on Days 1, 2, 4, 5, 8, 9, 11, and 12 of 21-day cycles) did not change during induction. In contrast, DEX lowered was defined as patients for whom the end-of-induction dex dose and/or frequency were lower than their starting values. As an exploratory analysis, we also compared PFS and OS between the DEX fullDose (no changes to dex strength during induction) and DEX veryLow (dex dosing reduced ≥50% during induction) subgroups. All analyses were landmarked from the end of induction, defined as 24 weeks from registration, to account for the time-dependent nature of dex treatment received. Survival curves were based on the Kaplan-Meier method and comparisons were made using the log-rank test.
Results: Of 541 evaluable patients ( n = 441 from S0777, n = 100 from S1211), dex was reduced during induction in 76% ( n = 410). Compared to DEX fullDose patients, DEX lowered patients were more likely to be anemic (37% vs 26%, p = 0.02) but had similar distributions of age ≥70 (24% of DEX lowered vs 26% of DEX fullDose), Performance Status > 1 (15% vs 12%), ISS stage 3 disease (33% vs 27%), ≥ 60% bone marrow plasma cell burden (37% vs 31%), M-spike > 3 g/dL (48% vs 48%) platelets < 150,000 cells/mm 3 (18% vs 14%), baseline LDH ≥ 190 U/L (36% vs 41%), and baseline CRP ≥ 8 mg/L (27% vs 30%).
PFS (Figure 1B) was comparable between DEX lowered (median 36 months, 95% CI 29 to 39 months) and DEX fullDose (median 33 months, 95% CI 24 to 48 months) with p = 0.90. Similarly, OS (Figure 1C) was comparable between DEX lowered [median 77 months, 95% CI 68 months to not reached (NR)] and DEX fullDose (median 92 months, 95% CI 65 months to NR) with p = 0.93. In the DEX veryLow subgroup with ≥50% reduction in dex dosing during induction, median PFS was 39 months (95% CI 34 to 50 months) and median OS was NR (95% CI NR to NR). As shown in Figure 1D and Figure 1E, PFS and OS were comparable between these patients and DEX fullDose patients.
Discussion: To our knowledge, this is the first analysis of dex dosing strength in NDMM since E4A03. In two separate randomized trials, over half of patients required dex reductions below a starting dose of ~40 mg weekly. Dex dose reductions during induction had no impact on post-induction PFS and OS, even in the subset of patients who required significant dose reductions. Limitations of our post hoc analysis include heterogeneity between dex dosing schedules and lack of cytogenetic data for all patients. We could not discern specifically why dex was lowered, and it is possible that dex might have been lowered disproportionately commonly in patients who had achieved early responses and thus independently did better. Regardless, these results strongly suggest that maintaining dex 40 mg weekly for the entirety of NDMM induction may be unnecessary in the modern era. Cooperative efforts to study a planned dex de-escalation protocol (i.e., once no longer needed for symptom management or as a pre-medication) alongside patient-reported outcome assessments of symptom burden are being planned.
Funding: NIH/NCI/NCTN grants U10CA180888, U10CA180819, U10CA180821, U10CA180820; and in part by Millennium Pharmaceuticals, Inc. (now part of Takeda Pharmaceutical Company), and by Celgene.
Disclosures
Banerjee:SparkCures: Consultancy; Sanofi: Consultancy; Genentech: Consultancy; Janssen: Consultancy; BMS: Consultancy; Caribou: Consultancy; Pfizer: Consultancy; Pack Health: Research Funding. Cowan:BMS, Adaptive: Consultancy; Adaptive Biotechnologies, Harpoon, Nektar, BMS, Janssen, Sano: Research Funding. Ailawadhi:AbbVie, Amgen, Ascentage, BMS, Cellectar, GSK, Janssen, Pharmacyclics, Sanofi: Research Funding; Beigene, BMS, Cellectar, GSK, Janssen, Pfizer, Regeneron, Sanofi, Takeda: Consultancy. Lonial:Janssen: Research Funding; TG Therapeutics Inc: Other: Board of Directors with Stock; Bristol-Myers Squibb Company, Janssen Biotech Inc, Novartis, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research, Research Funding; AbbVie Inc, Amgen Inc, Bristol-Myers Squibb Company, Celgene Corporation, Genentech, a member of the Roche Group, GlaxoSmithKline, Janssen Biotech Inc, Novartis, Pfizer Inc, Takeda Pharmaceuticals USA Inc: Consultancy, Other: Advisory Committee; Novartis: Research Funding. Usmani:EdoPharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Moderna: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hoering:Cancer Research And Biostatistics: Current Employment. Orlowski:Asylia Therapeutics: Current equity holder in private company, Patents & Royalties; Asylia Therapeutics, BioTheryX Inc., Heidelberg Pharma: Other: Laboratory Research Funding, Research Funding; BMS/Celgene Corporation, CARsgen Therapeutics, Exelixis Inc., Heidelberg Pharma, Janssen Biotech Inc., Sanofi/Genzyme, Takeda Pharmaceuticals USA Inc.: Other: Clinical Research Funding, Research Funding; AbbVie, Adaptive Biotech, Asylia Therapeutics, Inc., BioTheryX, Bristol-Myers Squibb Pharmaceuticals, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Nanjing IASO Biotherapeutics, Neoleukin Corporation, Oncopeptides AB, Pfizer, In: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal